How To Find How Many Valence Electrons
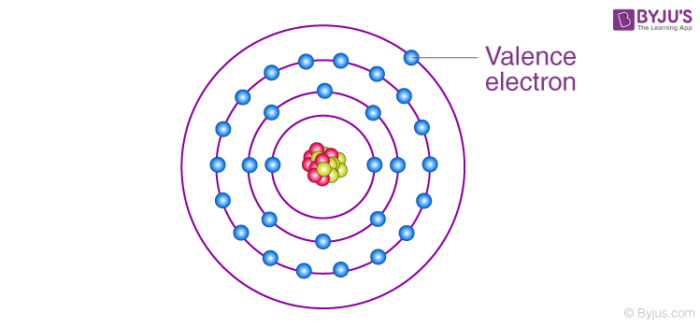
Valence electrons are the s and p electrons in the outermost shell. The electrons nowadays in the inner trounce are core electrons. When nosotros written report and observe the atom of an element, we come across tiny subatomic particles called valence electrons. Lewis structures help us to rail the valence electrons and predict the types of bond.
Valence electrons are all arranged in dissimilar orbitals or shells and are by and large negatively charged particles. Further, these electrons are responsible for interaction between atoms and the formation of chemical bonds. Notwithstanding, not all electrons are associated with the cantlet. Only the electrons present in the outermost shell tin participate in the germination of a chemic bail or a molecule. Such type of electrons is chosen valence electrons.
Table of Contents
- What are Valence Electrons?
- Related Videos
- Characteristics of Valence Electron
- Determination of Valence Electrons
- Valence Electron of Elements
- Frequently Asked Questions – FAQs
What are Valence Electrons?
Valence is the number of electrons an atom must lose or gain to accomplish the nearest noble gas or inert gas electronic configuration. "Electrons in the outer shells that are not filled are chosen valence electrons" .
The valence electrons are part of most of the chemic reactions because they contain more than energy compared to the electrons nowadays in inner orbits. Meanwhile, the number of valence electrons present as well helps usa decide a specific element's chemical properties, such as its valence or valency, the formation of bonds with other elements. It besides gives united states of america an idea of how readily the atoms can form bonds, the number of unpaired electrons and how many atoms tin have part.
Related Videos

Characteristics of Valence Electron
Electrons are involved in the chemical bonding and reactions of the atom. It is said to occupy orbitals in an atom. The number of valence electrons of an atom can be obtained from the periodic tabular array considering information technology is equal to the grouping number of the cantlet. Atoms are most stable if they have a filled valence shell of electrons. Atoms transfer or share electrons in such a way that they can attain a filled beat out of electrons.
Some cardinal characteristics of a valence electron are;
- For the main group elements, the valence electron exists only in the outermost electron shell.
- A valence electron can exist in the inner shell of a transition metal.
- An atom consisting of a airtight beat out of valence electrons volition usually exist chemically inert.
- A valence electron can either blot or release energy in the form of a photon.
- Valence electrons also determine the electrical conductivity of an chemical element. Depending on in this nature of elements tin be a metallic, non-metal, or a metalloid.
Conclusion of Valence Electrons
I of the easiest ways to find valence electrons is by checking out the elements' place in the periodic table. The valence electrons of an chemical element can exist found out by closely examining the vertical column in which the elements are grouped in. By looking at the group number that is given nosotros can identify the number of valence electrons that an element which is listed under that specific column has.

Another way to discover or determine valence electrons is by knowing the electronic configuration.
However, if we take the transition metals (groups three-12), finding the valence electron is quite complicated. These elements atomic construction is rigid and the d subshell is incomplete and sits lower than the outer trounce.
Valence Electron of Elements
Here is a list of the number of valence electrons present in the unlike groups.
| Periodic Tabular array Group | Valence Electrons |
| Brine metals – Group 1 (I) | i |
| Alkaline earth metals – Group ii (Two) | two |
| Boron group – Group 13 (Three) | 3 |
| Carbon group – Group 14 (Four) | 4 |
| Nitrogen grouping – Grouping fifteen (5) | 5 |
| Oxygen group – Grouping 16 (Half dozen) | 6 |
| Halogens – Grouping 17 (7) | seven |
| Noble gases – Grouping xviii (VIII or 0) | 8 |
Frequently Asked Questions – FAQs
How do you find the valence electrons?
For neutral atoms, the number of valence electrons is equal to the atom's chief group number. The primary group number for an element can be found from its cavalcade on the periodic table. For instance, carbon is in group 4 and has four valence electrons. Oxygen is in group 6 and has 6 valence electrons.
What is valence electron with example?
The total number of electrons present in final trounce orbit is known every bit valence electron. For case: Oxygen have 6 electrons nowadays in last orbital shell hence 6 is valence electron.
What is chosen valency?
Valency is the combining power of an element. Elements in the same group of the periodic table accept the same valency. The valency of an element is related to how many electrons are in the outer shell.
What is the purpose of valence electrons?
Valence electrons are the outer-shell electrons of an atom. Valence electrons decide the reactivity of an atom.
How do valence electrons work?
Valence electrons are the electrons located at the outermost beat of an atom. … Because when two atoms collaborate, the electrons in the outermost shells are the first ones to come into contact with each other and are the ones that make up one's mind how an atom will react in a chemical reaction.
To acquire more about this topic and other related topics, annals with BYJU'S and download the mobile application on your smartphone.
Source: https://byjus.com/chemistry/valence-electrons/#:~:text=For%20neutral%20atoms%2C%20the%20number,and%20has%206%20valence%20electrons.
Posted by: torrezandessaint.blogspot.com

0 Response to "How To Find How Many Valence Electrons"
Post a Comment